
Amino Acids as Chiral Anionic Ligands in Ruthenium Based Asymmetric Olefin Metathesis
Elisa Ivry, Chemistry, Ben-Gurion University of the Negev, Beer-Sheva, Israel
Amos Ben-Asuly, Chemistry, Ben-Gurion University of the Negev, Beer-Sheva, Israel
Gabriel Lemcoff, Chemistry, Ben-Gurion University of the Negev, Beer-Sheva, Israel
Olefin metathesis is a powerful synthetic tool in organic chemistry, allowing straightforward formation of carbon-carbon double bonds. Asymmetric olefin metathesis provides accessible pathways for the production of some elusive chiral products including complex natural products and stereoregular unique polymers [1]. Notwithstanding the efficiency observed for the known ruthenium chiral complexes, they all require intricate and expensive syntheses of chiral NHC ligands. Thus, exploration of straightforward and low cost synthesis protocols of novel functional group tolerant ruthenium pre-catalysts for asymmetric metathesis has remained highly desirable [2]. Additionally, due to usually required optimization, a tunable chiral catalysts is also a sought after goal.
As a first and novel approach to these challenges, we report the syntheses of Grubbs-Hoveyda type ruthenium pre-catalysts, bearing amino acids as chiral anionic ligands, and their application to asymmetric metathesis reactions.
[1] K. Grela, Ed.; Olefin Metathesis: Theory and Practice, John Wiley & Sons, 2014
[2] J. Tornatzky, A. Kannenberg and S. Blechert, Dalton Trans., 2012, 41, 8215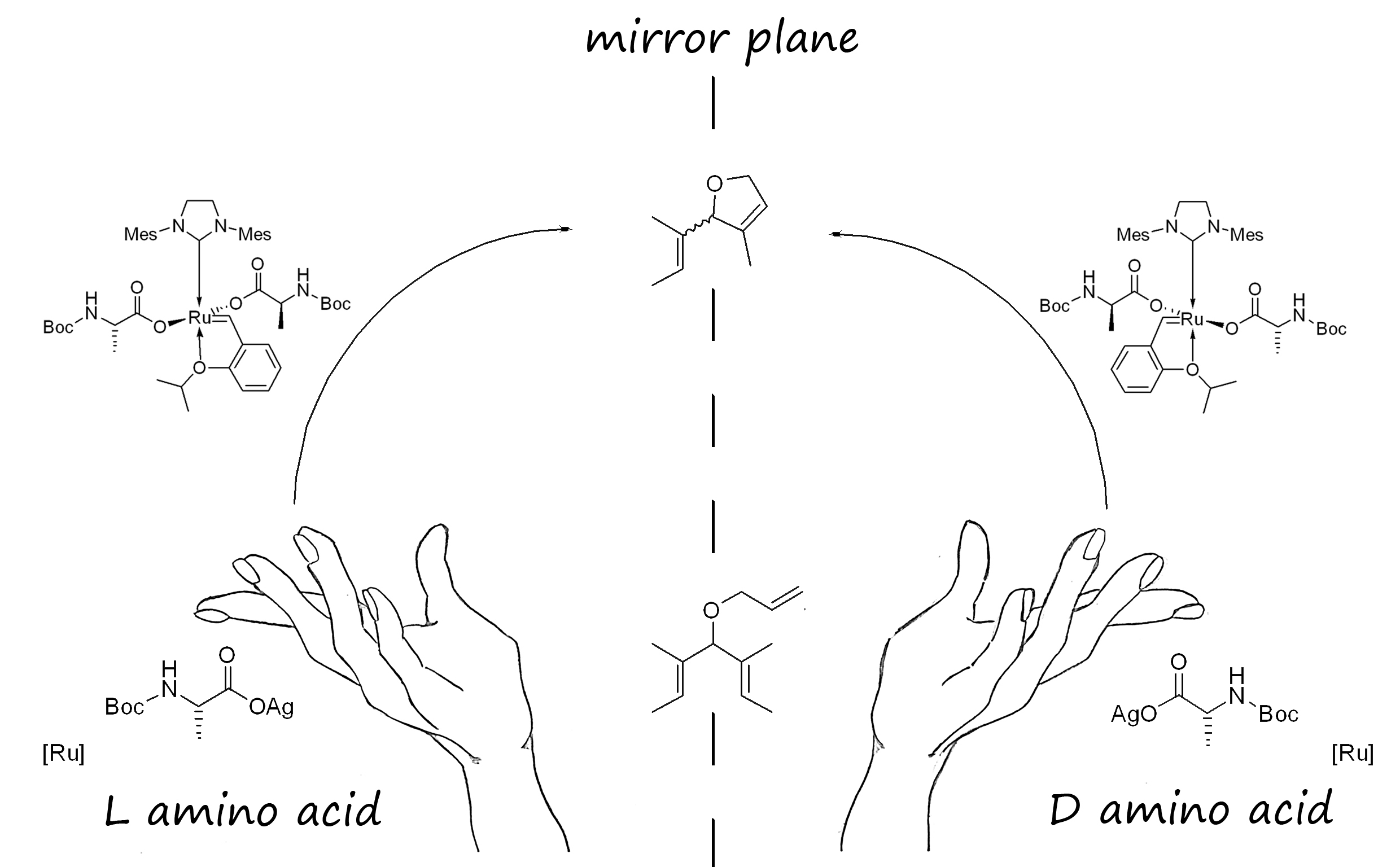
Organized & Produced by:

POB 4043, Ness Ziona 70400, Israel
Tel.: +972-8-9313070, Fax: +972-8-9313071
Site: www.bioforum.co.il,
E-mail: bioforum@bioforum.co.il