
A One-Pot Anodic Thiocyanation of Alkenes
Avishai Levy, Chemistry, Ben Gurion University of the Negev, Beer Sheva, Israel
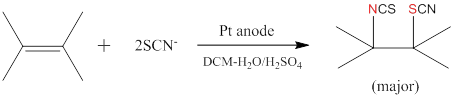
Electrochemical thiocyanation of alkenes was studied in an undivided cell using a one-pot, two-phase medium (water-dichloromethane) or one phase with two solvents (water-acetonitrile), employing a constant current electrolysis (CCE). The aqueous solvent is utilised to oxidise thiocyante anions, and the organic solvent is utilised to dissolve the alkene substrate and stabilise the electro-generated thiocyanogen (SCN)2 which is unstable in aqueous media. Thiocyanation was first implemented on a model alkene, the symmetric 2,3-dimethyl-2-butene, using a two-phase medium. The major product is a mixed di-addition of thiocyano and isothiocyano groups to the double bond. Other minor products involve addition of two thiocyano groups, one thiocyano and one hydroxy group, and a trace amount of one thiocyano group and a hydrogen atom.
When implementing the one phase – two solvents system (CH3CN-H2O) on the same substrate, the thiocyano and hydroxyl vicinal addition becomes more prominent.
After obtaining "optimal" conditions, other alkenes were examined.
Organized & Produced by:

POB 4043, Ness Ziona 70400, Israel
Tel.: +972-8-9313070, Fax: +972-8-9313071
Site: www.bioforum.co.il,
E-mail: bioforum@bioforum.co.il