
Cathepsin-C Inhibitors as a Mechanistic Probe and Drug Candidates for Necrotic Cell Death
Shira Hirsch, Chemistry, Bar-Ilan University, Ramat Gan, Israel
Amnon Albeck, Chemistry, Bar-Ilan University, Ramat Gan, Israel
Necrosis is associated with many clinical conditions, such as myocardial infarction, brain stroke, liver cirrhosis, trauma, or neurodegenerative diseases. It has been regarded as an unregulated and pathological process. However, recent studies indicate that necrosis might be a controlled and ordered event.
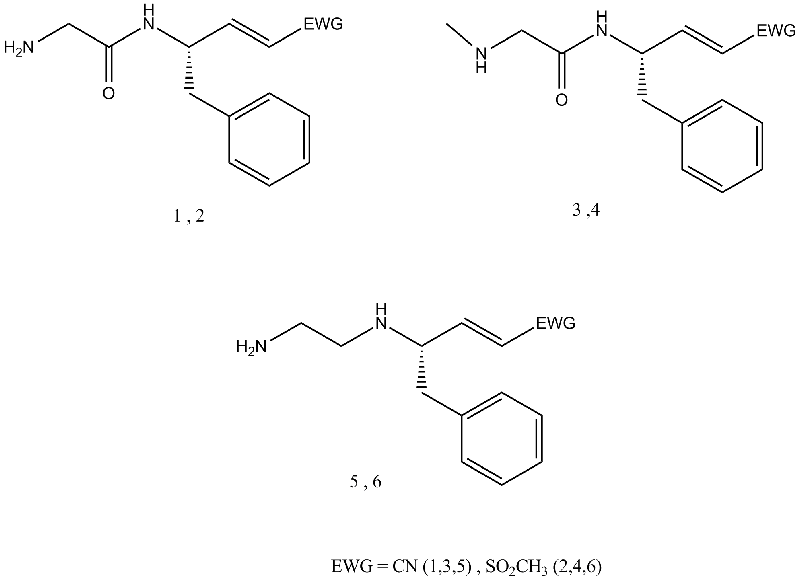
A collaborative research with Prof. Ilana Natan at Ben-Gurion University identified increased elastase-like proteolytic activity in cells exposed to necrosis inducers. This laid the grounds for the current study, dealing with the development of inhibitors against cathepsin C, an enzyme that is known to activate other proteases, including elasteses. Cathepsin C is a dipeptidyl amino peptidase, belonging to the cysteine proteases family. we synthesized both peptide-based (compounds 1-2) and pepetidomimetic-based (compounds 3-6) irreversible inhibitors against this enzyme (Fig 1).
The new compounds exhibited time- and concentration-dependent irreversible inhibition of the isolated enzyme, cathepsin C. The inhibitors ability to prevent necrosis was tested in two different in vitro systems: hypoxia of rat skeletal muscle cells, and exposure of PC-12 cells to KCN. Following the induction of necrosis, the level of cytoplasmatic enzymes ( LDH or CK ) in the supernatant was measured in order to determine the extent of necrotic cell death. The new inhibitors lead to up to 50% reduction of cell death.
This research provides new insight into the role proteases play in necrotic cell death, and points to a new potential approach for the treatment and prevention of necrosis.
Fig 1
Organized & Produced by:

POB 4043, Ness Ziona 70400, Israel
Tel.: +972-8-9313070, Fax: +972-8-9313071
Site: www.bioforum.co.il,
E-mail: bioforum@bioforum.co.il