
S-Chelated Ruthenium Alkylidenes: Expanding the Latency Gamut
Yakov Ginzburg, Chemistry, Ben Gurion University of the Negev, Beer Sheva, Israel
Eyal Tzur, Chemical Engineering, Shamoon College of Engineering, Ashdod, Israel
N. Gabriel Lemcoff, Chemistry, Ben Gurion University of the Negev, Beer Sheva, Israel
Olefin metathesis catalysts bearing o-tolyl-N-heterocyclic carbenes reported by Grubbs[i] and Grela,[ii] allow cross-metathesis of hindered olefins as well as ring-closing metathesis of olefins to afford tetra-substituted alkenes. We have expanded the scope of this reaction by adding the latency factor to the equation;[iii] achieving a new complex that is both latent at ambient conditions and extremely active upon heating. The new complex shows reactivity trends parallel to its non-latent analogues and latency similar to dormant complexes previously reported in our group. In addition, we have probed the effect of removing aromaticity from the chelating ring, disclosing its influence on stability and latency of the complexes.[iv]
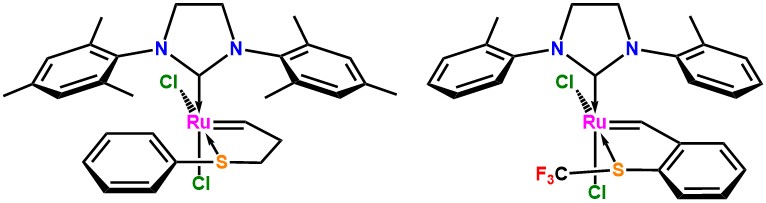
[i] Ian C. Stewart, Christopher J. Douglas, and Robert H. Grubbs, Org. Lett., 2008, 10, 441.
[ii] Christian Torborg, Grzegorz Szczepaniak, Adam Zielinski, Maura Malinska, Krzysztof Wozniak and Karol Grela, Chem. Commun., 2013, 49, 3188
[iii] a. Charles E. Diesendruck, Olga Iliashevsky, Amos Ben-Asuly, Israel Goldberg, N. Gabriel Lemcoff, Macromol. Symp. 2010, 293, 33.
b. Yakov Ginzburg, Aviel Anaby, Yuval Vidavsky, Charles E. Diesendruck, Amos Ben-Asuly, Israel Goldberg, N. Gabriel Lemcoff, Organometallics, 2011, 30, 3430.
Organized & Produced by:

POB 4043, Ness Ziona 70400, Israel
Tel.: +972-8-9313070, Fax: +972-8-9313071
Site: www.bioforum.co.il,
E-mail: bioforum@bioforum.co.il